- Published in Expert Opinion
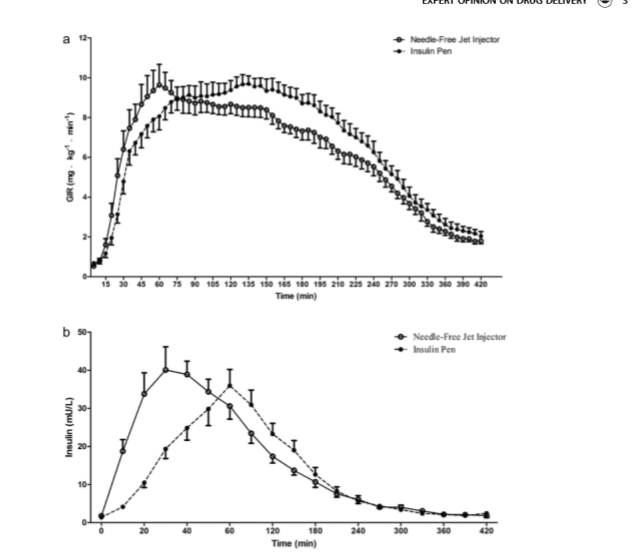
Lispro administered by QS-M needle-free injector results in earlier and higher insulin exposure than conventional pen, and a greater early glucose-lowering effect with similar overall potency.
Objective: The aim of this study is to evaluate the pharmacokinetic and pharmacodynamic (PK-PD) profiles of lispro administered by the QS-M needle-free jet injector in Chinese subjects.
Research design and methods: A randomized, double-blind, double-dummy, cross-over study was performed. Eighteen healthy volunteers were recruited. Lispro (0.2 units/kg) was administered by the QS-M needle-free jet injector or by conventional pen. Seven-hour euglycemic clamp tests were performed. Eighteen volunteers (nine men and nine women) were recruited in this study. The inclusion criteria were: nonsmokers aged 18–40 years, with body mass index (BMI) of 17–24 kg/m2; subjects with normal biochemical tests, blood pressure, and electrocardiograph; subjects who signed the informed consent. The exclusion criteria were: subjects with insulin allergy or other allergic history; subjects with chronic diseases such as diabetes, cardiovascular diseases, liver or kidney disease. Subjects who used alcohol were also excluded. The study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Results: A larger area under the curve (AUCs) of insulin concentration and glucose infusion rate (GIR) during the first 20 minutes after lispro injection by the jet injector compared to the insulin pen was observed (24.91 ± 15.25 vs. 12.52 ± 7.60 mg. kg−1, P < 0.001 for AUCGIR,0–20 min; 0.36 ± 0.24 vs. 0.10 ± 0.04 U min L−1, P < 0.001 for AUCINS, 0–20 min). Needle-free injection showed a shorter time to reach maximum insulin concentration (37.78 ± 11.14 vs. 80.56 ± 37.18 min, P < 0.001) and GIR (73.24 ± 29.89 vs. 116.18 ± 51.89 min, P = 0.006). There were no differences in total insulin exposure and hypoglycemic effects between the two devices. Conclusion: Lispro administered by QS-M needle-free injector results in earlier and higher insulin exposure than conventional pen, and a greater early glucose-lowering effect with similar overall potency.
Post time: Apr-29-2022
